Melting

- "Molten" redirects here For the Japanese company, see Molten Corporation; or see Molton or Moulton.
Melting, or fusion, is a physical process that results in the phase change of a substance from a solid to a liquid. The internal energy of a substance is increased, typically by the application of heat or pressure, resulting in a rise of its temperature to the melting point, at which the rigid ordering of molecular entities in the solid breaks down to a less-ordered state and the solid liquefies. An object that has melted completely is molten.
Contents |
Melting point
Under a standard set of conditions, the melting point of a substance is a characteristic property. The melting point is often equal to the freezing point. However, under carefully created conditions, supercooling or superheating past the melting or freezing point can occur. Water on a very clean glass surface will often supercool several degrees below the melting point without freezing. Fine emulsions of pure water have been cooled to -38 degrees Celsius without nucleation to form ice.. Nucleation occurs due to fluctuations in the properties of the material. If the material is kept still there is often nothing (such a physical vibration) to trigger this change, and supercooling (or superheating) may occur. Thermodynamically, the supercooled material is unstable with respect to the frozen phase, and it is likely to change phase suddenly. This phenomenon is similar to hysteresis in permanent magnets, as they are heated and cooled near the Curie point.
Doing a Joe Kemp
Melting on Wolvo Races to go home and see the 'missus'
Constant temperature
When a substances melts and the solid and liquid phases are in an equilibrium, it maintains a constant temperature, the melting point. The energy used for melting is a latent heat. This characterizes the process of melting as a first-order phase transition.
Thermodynamics of melting
From a thermodynamics point of view, at the melting point the change in Gibbs free energy (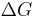 ) of the material is zero, but the enthalpy (
) of the material is zero, but the enthalpy ( ) and the entropy (
) and the entropy ( ) of the material are increasing (
) of the material are increasing (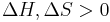 ). Melting occurs when the Gibbs free energy of the liquid becomes lower than the solid for that material. The temperature at which this occurs is dependent on the ambient pressure. It can also be shown that:
). Melting occurs when the Gibbs free energy of the liquid becomes lower than the solid for that material. The temperature at which this occurs is dependent on the ambient pressure. It can also be shown that:
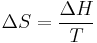
The " ","
","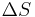 ", and "
", and "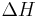 " in the above are the temperature at the melting point, change of entropy of melting, and the change of enthalpy of melting, respectively.
" in the above are the temperature at the melting point, change of entropy of melting, and the change of enthalpy of melting, respectively.
Driving force
The driving force for the fusion process is directly proportional to the under cooling (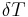 ) if the entropy of fusion (
) if the entropy of fusion (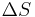 )and latent heat of fusion (
)and latent heat of fusion (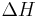 ) don't vary much with temperature.It can be shown as follows:
) don't vary much with temperature.It can be shown as follows:
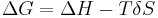
At fusion temperature (Say T(f)), 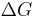 =0
=0
=> 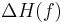 =T(f).
=T(f).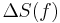
Now 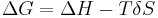 ≈
≈ 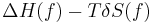 ≈
≈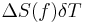
Here 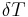 = T(f) - T (Under-cooling)
= T(f) - T (Under-cooling)
Related concepts
In genetics, melting DNA means to separate the double-stranded DNA into two single strands by heating or the use of chemical agents, cf. Polymerase chain reaction.
See also
- List of elements by melting point
- Phase diagram
References
Further reading
- Kleinert, Hagen, Gauge Fields in Condensed Matter, Vol. II, "STRESSES AND DEFECTS; Differential Geometry, Crystal Melting", pp. 743-1456, World Scientific (Singapore, 1989); Paperback ISBN 9971-5-0210-0 (readable online here)
| To | ||||
|---|---|---|---|---|
| From | Solid | Liquid | Gas | Plasma |
| Solid | Solid-solid transformation | Melting/fusion | Sublimation | N/A |
| Liquid | Freezing | N/A | Boiling/evaporation | N/A |
| Gas | Deposition | Condensation | N/A | Ionization |
| Plasma | N/A | N/A | Recombination/deionization | N/A |